Research lines Brain Plasticity Group
Structural and Functional Plasticity of the Nervous System
-
Dr H.J. (Harm) Krugers - Understanding how (early life) stress alters brain and behaviour
In our daily lives, we are regularly exposed to stressful and emotionally arousing events. While most people can adapt well to such events, some individuals are at risk for developing stress-related disorders, like major depression, anxiety disorders and post-traumatic stress-disorder.
Coping with stressful events requires appropriate cognitive processing in order to promote behavioural adaptation. The overarching goal of our lab is to understand how individuals adapt to stressful and emotionally arousing events and how resilience and vulnerability to develop stress-related disorders is determined.
Our research can roughly be divided into three themes:- Understand how stress and stress-hormones modulate learning, memory and fear
- Understand the mechanisms how stress early in life determines learning and memory and fear over the life span and over generations;
- Understand the mechanisms how early life experiences affect social behavior later in life.
To address these questions, the Krugers lab exploits and combines animal behavior with electrophysiology, biochemical tools, pharmacology, molecular tools and cellular imaging. More recently, the lab also performs translational studies on fear and fear generalization in humans and healthy aging in humans.
Harm Krugers is Associate Professor at SILS. He is Program Director of the Research Master Brain and Cognitive Sciences, board member of Amsterdam Brain and Cognition (ABC); Board member of the Society for Interdisciplinary Behavioural Research (SIGO) and member of the executive board of FENS.
-
Dr A. (Aniko) Korosi - Programming of mental and metabolic health by early-life stress focus on inflammation and nutrition
Research aims
Early life is a sensitive period of development. When this period is disturbed by exposure to childhood adversity, it can have a lasting impact on the adult we become, on mental and physical health, increasing the vulnerability to develop a large range of disorders (e.g. depression, cognitive decline and obesity). Because prevention of early-life stress is often difficult, a better understanding of the mechanisms that underlie the early programming of the brain, behavior and body is needed.
We study the effects of early-life stress in the context of wild type, but also Alzheimer mouse models. We have recently found that exposure to early-life stress is associated with impaired learning and memory, altered neuroimmune system, altered responses to amyloid and altered fat and leptin system.
Importantly, we have recently identified early nutritional interventions with essential PUFAs or with methyl donor micronutrients, that were able to protect against some of the detrimental effects of early-life stress exposure. The exact mechanisms underlying the beneficial effects of such diet, and whether this could be considered also in the context of Alzheimer’s disease, are among our current research interests.
The main goal of our research group is to better understand the biological mechanisms and environmental factors involved in brain programming by stressful early-life experiences and to test the efficacy of nutritional interventions. We focus on essential nutrients and early dietary interventions in particular. The translational value of this approach is high as nutrition is typically non-invasive, cheap and easily applicable.
We aim to identify how the various components of the early-life environment, including stress-hormones, early nutrition and inflammatory modulators act synergistically in programming the brain and body.
We study these aspects across species, in mice, humans and fish.Mice
We use wild type as well as transgenic mice (e.g. Alzheimer’s disease mouse model) and an established mouse model of early-life stress. We study if and how early-life stress-induces alterations in brain structure and function and metabolic characteristics under a basal state or after various challenges (e.g. immune challenge, or metabolic challenges) and study in detail the involvement of various cell types of the brain (neurons, astrocytes, microglia), in vivo and in vitro. Within the brain, our main focuses are on the hippocampus and hypothalamus, very plastic brain regions involved in learning and memory, in stress regulation and energy homeostasis, and focus on various forms of brain plasticity, related to hippocampal neurogenesis, microglia and astrocytes.Human
- We have set up the Amsterdam mother milk cohort (AMS) to study the effects of stress on the composition of human breastmilk, in collaboration with Prof Hans van Goudoever (AUMC).
- We work in collaboration with AUMC (Dr. S. de Rooij) on the Dutch Famine birth cohort and ABCD cohorts to understand the biological characteristics (lipid and immune profile) of blood, aimed at understanding mechanisms and possibly identifying biomarkers of early stress exposure.Fish
Work in collaboration with WUR (Dr. B. Pollux) on novel placental fish species to study the evolution of maternal effects on the offspring.If you are interested in more details, my personal website is: https://akorosi.wixsite.com/korosigroup
Aniko Korosi is Associate Professor at SILS. She is coordinator of the Neuroscience master track (PPP, Psychopathophysiology) of the Research Master biomedical Sciences, treasurer and member of the executive board of the European brain and behavior society (EBBS), member of the executive board of the Dutch Neuroscience meeting (DNM) and chair of DNM2020/2021. Member of the ECNP nutrition network, Aniko Korosi is member of the editorial board of several recognized journals, including eNeuro and Frontiers in Neuroenergenetics, Nutrition and Brain health, and she regularly acts as evaluation and advisory board member for several national and international grant agencies and research councils.
-
Dr C.P. (Carlos) Fitzsimons - Common molecular mechanisms of brain insults affecting the hippocampus
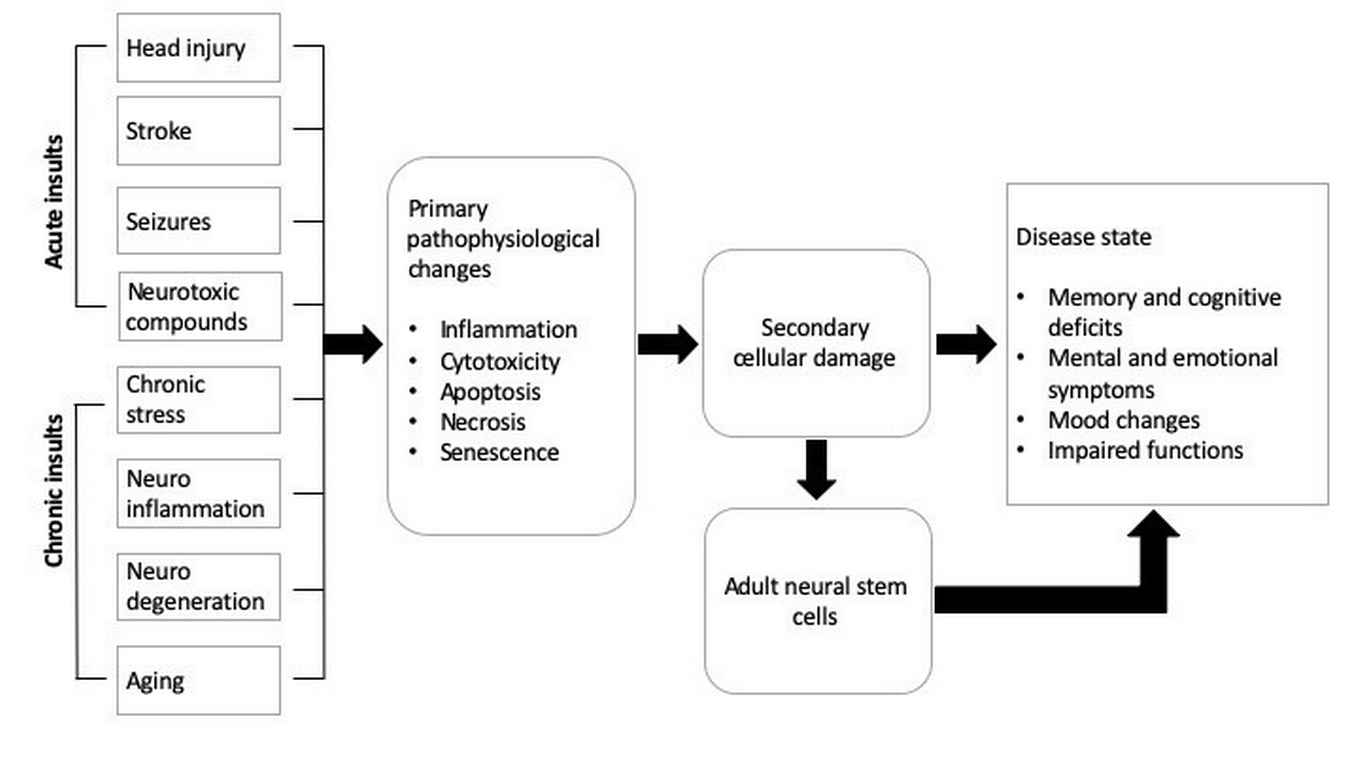
Our central hypothesis is that a group of common brain-damaging insults such as concussions and head injuries, stroke, epileptic seizures, chronic stress, neuroinflammation, neurodegeneration, aging, et cetera, modify the proliferation capacity and other key biological functions in neural stem cells present in the adult brain, thereby contributing to the cellular changes associated with brain disease and affecting cognition.
The Fitzsimons Lab has been financed by the Innovational Research Incentive Scheme VIDI from The Netherlands Organisation for Scientific Research (NWO), the International Foundation for Alzheimer's Research (ISAO) and Alzheimer Nederland and by ERA-NET NEURON. The Network of European Funding for Neuroscience Research (NEURON) is a European Research Area Network (ERA-NET) co-funded by the European Commission that supports basic, clinical and translational research in the field of disease-related neuroscience. As a co-fund, this ERA-NET NEURON project receives local financial support from NWO/ZonMw, Hersenstichting Nederland, the National Initiative Brain and Cognition and is part of the Topsector Life Sciences & Health program.
Detailed information about our past and ongoing activities, experimental approaches used in the lab and scientific expertise can be found at our lab’s website: https://www.fitzsimonslab.eu/
Current international collaborations include:
- Astrogliosis and neuroinflammation: Matthew Holt, Lab of Glia Biology, VIB-KU Leuven Center for Brain and Disease Research, and Adrian Liston, Babraham Institute, Cambridge, UK.
- Biology of small non-coding RNAs: Davide de Pietri Tonelli, Italian Institute of Technology, Genoa, Italy.
- Contribution of reactive neural stem cells to hippocampal pathology: Juan Manuel Encinas, Achucaro Basque Center for Neuroscience, Bilbao, Spain.
Carlos Fitzsimons is member of the editorial board of several recognized journals, including Scientific Reports, Frontiers in Neuroscience, Frontiers in Cellular Neuroscience and Frontiers in Molecular Neuroscience and he regularly acts as evaluation and advisory board member for several European and international research councils.
-
Dr J.D. (Joram) Mul - Exercise, brain plasticity and stress robustness (Fit Brain lab)
What do we do?
Our main goal is to elucidate adaptations in the brain that underlie the beneficial effects of physical activity/exercise on stress robustness and cognition.
Why do we do this?
Elucidating mechanisms by which repeated physical activity (i.e. exercise training) promotes stress robustness, the brain’s ability to cope with prolonged stress exposure while maintaining normal psychological functioning, is a major research challenge given the high prevalence of stress-related mental disorders, including depression. Exercise training can also impact cognition, and elucidating the underlying mechanisms can lead to new therapies to improve cognition when impaired or halt age-related cognitive decline.
How do we do this?
We perform both fundamental and human studies, resulting in a unique translational approach that increases our knowledge of the beneficial brain adaptations during physical activity and/or exercise training.
On the fundamental side, we use voluntary wheel running (VWR) to gain mechanistic insight how voluntary physical activity produces adaptations in brain regions that modulate stress robustness and cognition. To do this, we use a wide variety of molecular techniques (including but not limited to RT-qPCR, Western Blot, immunohistochemistry, RNA sequencing, and synaptic function analysis). Our interests include determining the functional consequences of VWR on adult hippocampal neurogenesis.
On the human side, we use wearable technology (e.g. wrist-worn accelerometers) to assess the role of physical activity in the development and/or relapse of stress-related psychiatric disorders. To do this, we focus on a network analysis approach. We also use functional Magnetic Resonance Imaging (fMRI) to visualize the impact of behavioral treatment, including exercise training, on brain function in subjects with stress-related psychiatric disorders.
Our work has received financial support from:
- Urban Mental Health institute
- Amsterdam Neuroscience
- Dr. J.L. Dobberke Foundation
- Amsterdam Brain and Cognition
- Nationale Wetenschapsagenda
-
Dr R. (Rixt) van der Veen - Impact of early life conditions on coping later in life
I am intrigued by the effects of environmental influences during development on later life (social) behaviour. In our group, we investigate this at several levels.
Thriving in life: Starting point of our studies
Thriving in the ‘jungle of life’ demands a specific set of (social) skills, for which the foundations are laid early in life. Their development can be strongly influenced by environmental conditions that are encountered. These early life conditions -that can vary in stress level- prepare an organism for the life ahead by shaping its stress and immune systems, brain functions and (social) behaviour. While this is mostly adaptive and helps us cope with our environment, strong stressors or harmful external factors may hinder a healthy development and lead to maladaptive behaviour, especially in challenging situations. However, not everyone is equally sensitive to these factors, and their impact is more obvious in individuals with a susceptible genetic background. We address these questions within the Consortium on Individual Development
Early life and Adolescence
Early postnatal life, and parental care within that period, is crucial for developing long-lasting set-points in the stress and reward systems. As adolescence represents a period in which brain structures mature, specifically those involved in social behaviour and behavioural control, the brain is also relatively vulnerable to disturbances at this stage of life. Both positive and challenging experiences in these early life sensitive periods shape adaptive behavior and might even interact or counteract each other’s effects. However, extended early life stress but also external factors like substance abuse during the adolescent period, can harm a healthy development of the brain. In our research, we model both negative and positive experiences during these developmental periods and study their impact on adult social behavior and brain function.
Urban mental health, modelling the demands of a complex society.
In urban settings, with its high population densities, the environmental load can be even higher, while also air, light and noise pollution, work pressure and extended access to all sorts of substances and incentives, can influence brain functions and mental health. Also here, we are not all equally well equipped to deal with this and a better understanding of the factors that influence successful coping in a demanding, urban environment, might help guide targeted interventions and improve mental health outcomes. As part of the Centre for Urban Mental Health, our lab models complex environments and studies the impact of disturbances of the early life and adolescent period on later functioning in a complex environment. We focus on environmental enrichment, social behaviour, drug exposure and decision making.
Open science
We are actively supporting open science initiatives, joining forces to make science in our research field accessible and re-usable:
Find MaBapp and run tailor-made meta-analyses for ELS models, revealing the optimal choice of experimental protocols and study power
Contact me for internship opportunities.
Selected key references
Van der Veen R, Bonapersona V, Joëls M (2020). The relevance of a rodent cohort in the consortium on individual development. Dev Cogn Neurosci. doi.org/10.1016/j.dcn.2020.100846.
Kentrop J, Kalamari A, Danesi CH, Kentrop JJ, van IJzendoorn MH, Bakermans-Kranenburg MJ, Joëls M, van der Veen R (2020). Pro-social preference in an automated operant two-choice reward task under different housing conditions: Exploratory studies on pro-social decision making. Dev Cogn Neurosci. Jul 18;45:100827. doi: 10.1016/j.dcn.2020.100827.
Knop J, van IJzendoorn MH, Bakermans-Kranenburg MJ, Joëls M, van der Veen R (2020). Maternal care of heterozygous dopamine receptor D4 knockoutmice: Differential susceptibility to early-life rearing conditions. Genes Brain Behav. Apr 18:e12655. doi: 10.1111/gbb.12655
Knop J, van IJzendoorn MH, Bakermans-Kranenburg MJ, Joëls M, van der Veen R (2019). The effects of different rearing conditions on sexual maturation and maternal care in heterozygous mineralocorticoid receptor knockout mice. Horm Behav. 112:54-64. doi: 10.1016/j.yhbeh.2019.04.001.
Kentrop J, Smid CR, Achterberg EJM, van IJzendoorn MH, Bakermans-Kranenburg MJ, Joëls M, van der Veen R (2018). Effects of maternal deprivation and complex housing on rat social behavior in adolescence and adulthood. Front Behav Neurosci. 12:193. doi: 10.3389/fnbeh.2018.00193.
Knop J, Joels M, van der Veen R (2017). The added value of rodent models in studying parental influence on offspring development: opportunities, limitations and future perspectives. Current Opinion in Psychology 15:174-181. doi: 10.1016/j.copsyc.2017.02.030.
Kentrop J, van der Tas L, Loi M, van IJzendoorn MH, Bakermans-Kranenburg MJ, Joëls M, van der Veen R (2016). Mifepristone treatment during early adolescence fails to restore maternal deprivation-induced deficits in behavioral inhibition of adult male rats. Front Behav Neurosci. 15(10):122. doi: 10.3389/fnbeh.2016.00122.
van der Veen R, Kentrop J, van der Tas L, Loi M, van IJzendoorn MH, Bakermans-Kranenburg MJ, Joëls M (2015). Complex Living Conditions Impair Behavioral Inhibition but Improve Attention in Rats. Front Behav Neurosci. 24(9):357. doi: 10.3389/fnbeh.2015.00357
van der Veen R, Boshuizen MC, de Kloet ER (2013). Mifepristone treatment affects the response to repeated amphetamine injections, but does not attenuate the expression of sensitization. Psychopharmacology(Berl). Dec;230(4):547-56. doi10.1007/s00213-013-3176-8.
Koehl M, van der Veen R, Gonzales D, Piazza PV, Abrous DN (2012). Interplay of maternal care and genetic influences in programming adult hippocampal neurogenesis. Biol Psychiatry72(4): 282-9. doi: 10.1016/j.biopsych.2012.03.001.
van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, Deroche-Gamonet V (2008). Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner.PLoSOne3(5): e2245. doi: 10.1371/journal.pone.0002245.
van der Veen R, Abrous DN, de Kloet ER, Piazza PV, Koehl M (2008). Impact of intra-and interstrain cross-fostering on mouse maternal care. Genes, Brain and Behavior7(2): 184-92. doi:10.1111/j.1601-183X.2007.00337.
van der Veen R, Piazza PV, Deroche-GamonetV (2007). Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology (Berl)193: 179-186. doi: 10.1007/s00213-007-0777-0.