Genes regulating the architecture of plant inflorescence
Genes regulating the architecture of plant inflorescence
The most important questions in modern biology are (1) How do we think? (2) How do we develop from a single cell? (3) How did we evolve? Obviously humans are not the only organisms that think, develop and evolve. Therefore answers to these complex questions are more likely to be found by studying very simple organisms such as yeast, the fruitfly, the nematode C. elegans or even plants.
Both animals and plants develop from a single fertilized egg cell into a complex structure with all organs and tissues in the right place. One of the fundamental questions in developmental biology is a how a "dumb thing" like a single cell can achieve this miracle.
Why comparative developmental biology (“Evo-devo”)?
The finding that master genes that regulate the development of the fruit fly are conserved in mammals (including humans) and play a very similar role in mammalian development came initially (in the mid 1980s) as a big surprise. Since then many more examples of such conservation of genes and their function have been discovered that it is today seen as the rule rather than the exception.
Yet, one does not have to be a biologist to see that species are actually very different from each other, not only in size, but also in their structural design or body architecture. Although we have some idea of the developmental mechanisms that are conserved between distinct species, we know little or nothing about the mechanisms that make them look so different. Because distinct species and body architectures arose from each other by evolution, it seems likely that many of the genes that dictate their body architecture are conserved and that the diversification results from alterations in only a few of those genes. The identity of those genes and the nature of gene products, how they got altered and how that affected the evolution of the body plan is, however, still a mystery.
This question is not easily addressed by studying animal development. Although, the finding that “homeotic genes” specifying the identity of body segments are conserved in species as divergent as flies and mammals became a paradigm, such comparative studies are unlikely to solve why insects and mammals have such different body architectures. One major difficulty is that the differences between a fly and a mammal are way too large to even consider trying to change a fly into a mammal by adjusting the expression or function of key genes. Alternatively comparative genomics studies of mouse and man may on the long term be helpful to unravel, for instance, the differences in development and functioning of the brain, but it will provide little insight into the evolution of body plan, because mouse (or other mammals) and man are too similar in this respect (each having 4 limbs, a head between the two fore legs etc.).
In contrast to animals, even closely related plants species can display an enormous variation in body plan, which is most dramatically seen in their inflorescences, the structure that carries the flowers (for some examples see Fig 1). In some species the inflorescence consists of only a single solitary flower, whereas other species generate multi-flower inflorescences in which the flowers are arranged in distinct patterns. Also the architectures of the flowers themselves can differ extensively with respect to the arrangement and numbers of floral organs. Because flowering plants are relatively closely related and because it is so easy to pinpoint homologous organs (leaves, flowers, sepals, petals etc), even in species as widely diverged as monocots and dicots, they make a very suitable model to study the molecular and evolutionary mechanisms that gave rise to the astonishing variety of plant shapes and architectures.
Genetic control of inflorescence and flower architecture
The development of the plant body depends on the activity of sets of undifferentiated cells at the apex (the shoot apical meristem or SAM) and the root tip (the root meristem). During plant development, these meristems generate new secondary meristems and primordia (the initials of organs) in well defined positions which subsequently adopt a certain fate or identity. The development of a petunia flower, for instance, requires the formation of four concentric whorls, each consisting of multiple primordia in well defined relative positions, that develop -from outside to inside- into sepals, petals, stamens and carpels respectively.
Although considerable progress has been made in elucidating the mechanisms that determine organ or meristem identity, we still know very little about the mechanisms that determine that primordia arise in defined positions.
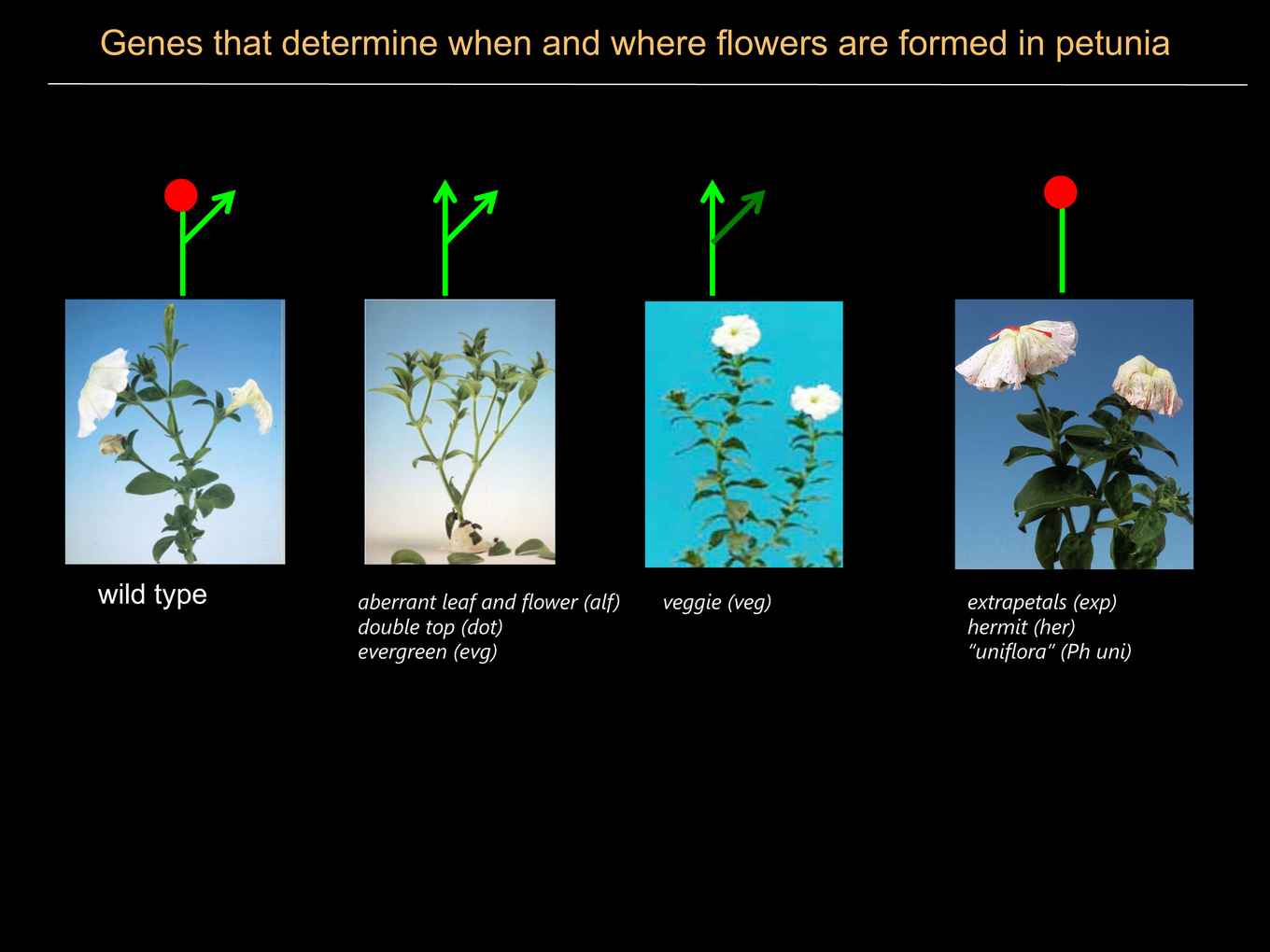
Wild type petunia has a so-called cymose inflorescence that contains an indeterminate number of flowers. Detailed microscopical studies showed that this architecture is generated by bifurcations of the apical inflorescence meristem, after which one half develops as an (determinate) floral meristem that will subsequently generate floral organ primordia, while the other half remains an inflorescence meristem and will undergo a new bifurcation to generate the next flower. By transposon mutagenesis we isolated several mutants in which the inflorescence structure is altered. These mutants fall in roughly two categories: meristem identity mutants and branching mutants.
Meristem identity mutants
The genes aberrant leaf and flower ( alf), double-top ( dot) and evergreen ( evg) are all required to specify the identity of floral meristems. In the absence of alf, dot or evg, flowers are replaced (transformed) by inflorescence shoots. ALF and EVG encode transcription factors, whereas DOT encodes a so-called F-box protein that is part of an SCF-type of ubiquitin ligase complex.
Branching mutants
In transposon mutagenesis experiments we identified mutants for three distinct loci -named EXTRAPETALS (EXP), HERMIT (HER) and VEGGIE (VEG)- in which the multiflower cymose inflorescence is replaced by a solitary flower (= “tulip-type” of inflorescence). This suggests that exp, her and veggie mutants all have a problem in the bifurcation of the inflorescence apex. Current research focuses on unraveling the mechanism by which EXP, HER and VEGGIE control development of the petunia inflorescence and the role of putative EXP homologs in species with a distinct inflorescence.