Flower pigmentation as model for the study of cellular processes
Flower pigmentation as model for the study of cellular processes
Once cells become committed to a specific developmental program, they start to differentiate and become visibly different from each other; which involves the specific activation of several 1000's of cell- and tissue-specific genes. To study how cell differentiation and pattern formation takes place, resulting ultimately in the activation of defined sets of genes, we focus on genes that are involved in anthocyanin synthesis. These pigments are accumulated in specialized cells (epidermis) on the surface of flower petals. Any mutation affecting the genes involved in their synthesis or in the characteristics of the cells accumulating them, results in an easy to spot change of colour. In this way we have identified the genes controlling the synthesis deposition of anthocyanins in specific organs and at specific moments. A group of regulatory genes encodes for transcription factors, forming a transcription complex required to “switch on” the genes for the enzymes directing the biosynthesis of the pigments. Such complex, called WMBW complex (from the names of the transcription factors taking part to it), is specifically expressed only in the cells of the petal epidermis.
Recently, the characterization of blue flower colour mutants brought us to discover a novel mechanism of acidification of the lumen of cellular compartments. The colour of petals is determined by the pH of the compartment in which the pigments are stored. If the pH becomes higher, the colour shifts towards blue, while a rather acidic environment results in reddish colours. These are necessary to attract pollinators and guarantee a numerous progeny to the plant. Thanks to the combined action of two P-ATPases (PH1 and PH5) petal epidermal cells can build a large pH gradient between the cytoplasm and the vacuolar lumen, allowing the flower to display a brilliant reddish colour. The expression of PH1 and PH5 is controlled by the WMBW complex. This hyperacidification mechanism was until now unknown also due to the difficulty to isolate mutants in other systems.
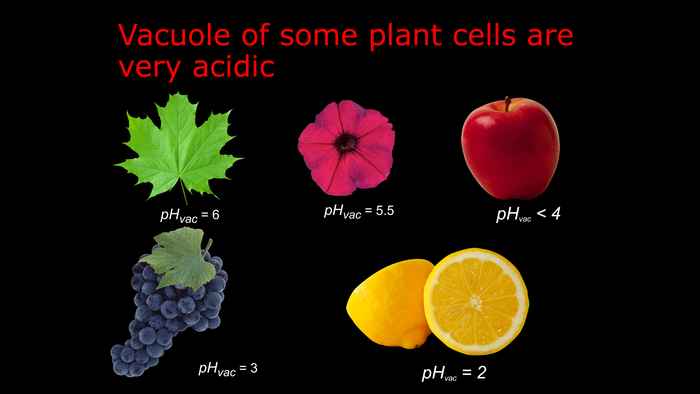
By studying the genes that in petunia control the conditions in the lumen of the vacuoles of the epidermis of petunia petals, we could unravel the mechanism that in citrus juice cells determine the extremely acidic environment (pH~2) and we identified the mutations that have been selected by breeders to isolate sweet tasting varieties of oranges, mandarins and other fruits.
Also the stabilization of the pigments in the vacuole is controlled by the WMBW complex, as shown by the “fading” of the colour in the petal of mutants for some of the transcription factors within the complex. We use these mutants to identify factors involved in the stabilization of the vacuolar content.
Genetics of vacuolar genesis and protein sorting
Among the peculiarities of the cells in the epidermis of petals, is a novel organelle, resembling a small vacuole and serving as “intermediate station” for proteins on their way to the central vacuole. This compartment, which we called vacuolino, is absent in cells that do not express the WMBW complex. As mutants, lacking organelles are scarce (due to their lethality in most systems), these plants offer the unique possibility to identify genes involved in the genesis of vacuolar compartments and the regulation of their functions.
The isolation of mutants in which vacuolinos are missing, allows to study the process by which these organelles are formed and their function. In the recent research of our group, we described a novel protein sorting pathway in which these organelles play a crucial role as check point. Proteins are checked in vacuolinos and if these do not ‘pass the test’ are degraded in these organelles and do not reach the central vacuole.
The protein responsible for the degradation of anthocyanins in ‘fading’ mutants, is in wild type petals halted in the vacuolinos, while where these are missing it arrives to the central vacuole resulting in the disappearance of color. The studies done in petunia found applications in other species (both ornamentals and agricultural crops) where maintenance of color is an important trait marketing trait.