Method published for the generation of bright red fluorescent proteins
15 January 2020
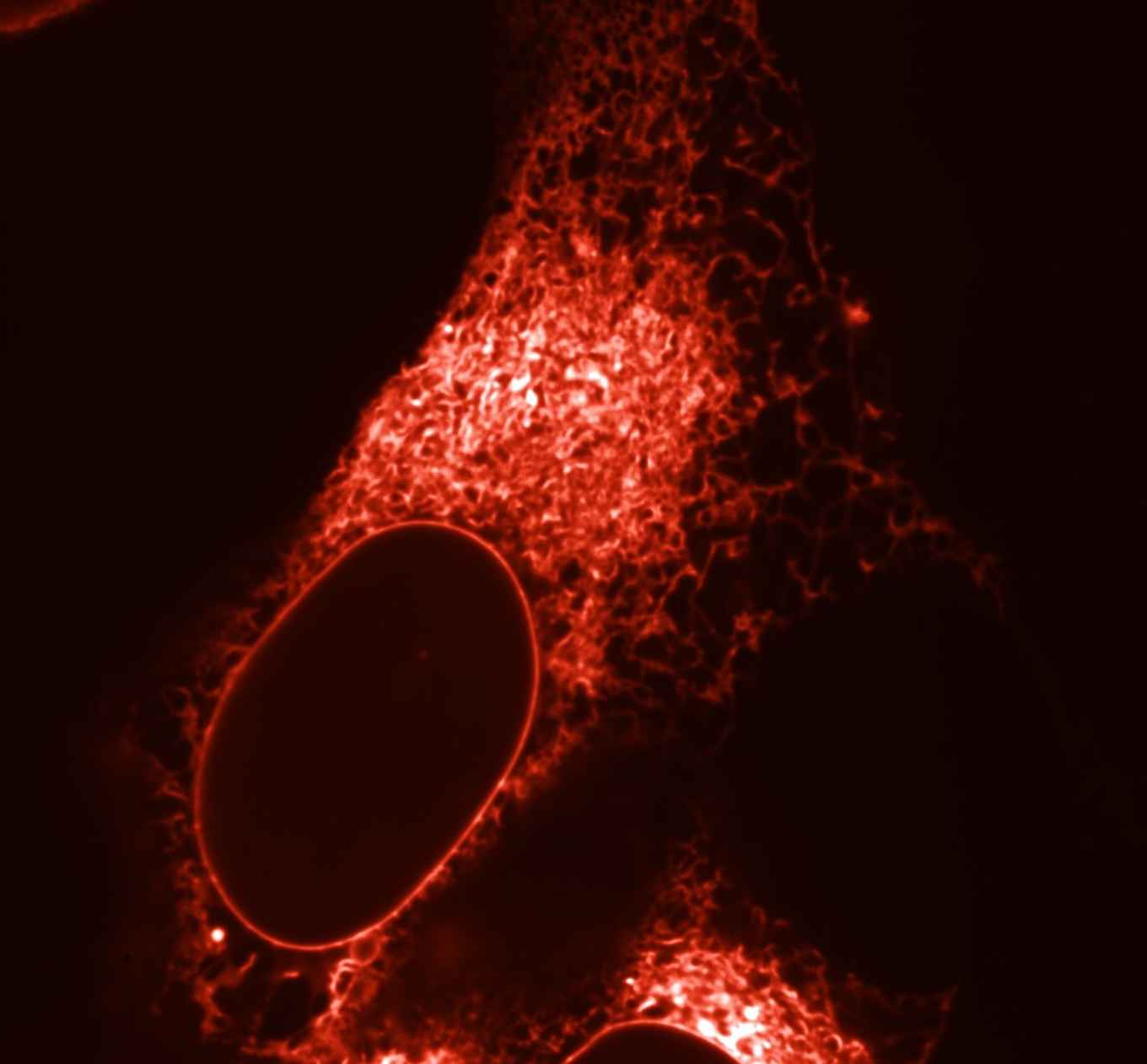
Fluorescent proteins have revolutionized cell biology by enabling researchers to visualize the dynamics of living cells at the molecular level with microscopy. Fluorescent proteins can be coupled to endogenous proteins within cells and as a result they will light up in the fluorescence microscope so that their behaviour and exact location within the cell can be monitored.
Meanwhile many differently coloured fluorescent proteins are available and by using multiple colours and coupling them to different structures and proteins, several processes can be monitored simultaneously within living cells. Until recently however, red fluorescent proteins were suboptimal because they formed aggregates in living cells and because they matured slowly or remained rather dim. Hence, these properties left substantial room for improvement.
Since red fluorescent proteins are fully genetically encoded in the DNA, many variants can be made by altering this genetic code. In this way billions of different red fluorescent protein variants can be easily made. However, this poses several problems: how to choose the best variant among these billions of variants, and how do you know that no better variant can be made? The largest problem is how to find a variant improved in not one or two properties but in all aspects? For example: bright red fluorescent proteins were already available, but they maturated very slowly and formed aggregates. While also very fast-maturating red fluorescent proteins have been made that did not aggregate but these where rather dim.
This challenge was addressed by UvA-biologists, for which they developed a method, which is now published by the research group of Molecular Cytology led by prof. Dorus Gadella. This method consists of a “multiparameter screening process” in which multiple properties of a large number of red fluorescent protein variants are determined simultaneously. This is achieved within living cells with the use of automized fluorescence microscopes and digital image processing. In their paper, Daphne Bindels, Marten Postma, Lindsay Haarbosch, Laura van Weeren and Dorus Gadella describe in a sort of cookbook recipe consisting of 75 steps what the right microscopy settings are and how the digital images should be processed in order to select the overall best variants. This method led to the creation of mScarlet, a very bright red fluorescent protein (published in Nature Methods).
Besides a detailed protocol also all software code has been made available to the scientific community to enable automatic screening of the best variants. With this protocol Gadella expects that other research groups worldwide also will be able to develop strongly improved fluorescent proteins and fluorescent biosensors to further boost cancer- and (stem) cell biology research.