Evolutionary Neurogenomics
Group leader dr. Frank Jacobs
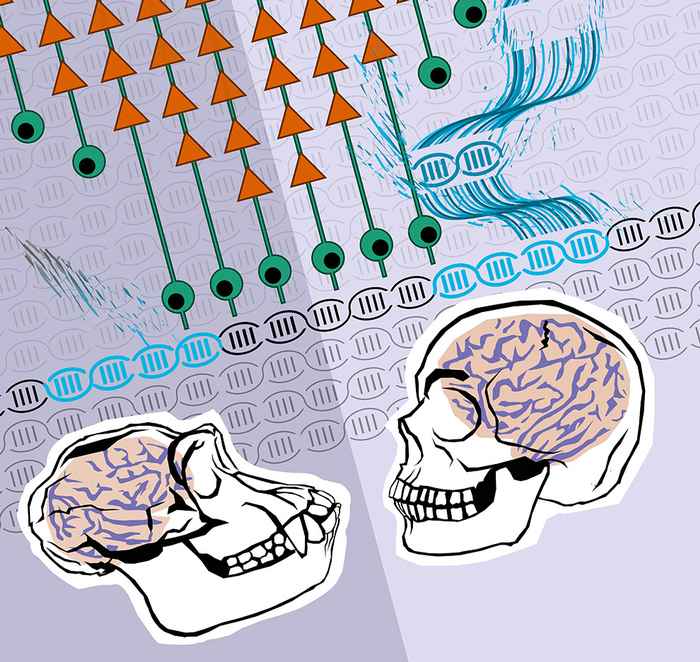
Genomic changes can be small, such as retrotransposon insertions, or big, such as segmental duplications of whole segments of the genome. Genomic modifications happened frequently during primate evolution, but the extent to which individual evolutionary genomic events accounted for changes in gene expression and contributed to the evolution of species remains unclear. The impact of both types of evolutionary genomic changes on human brain development and disease are the main topics in my lab's research. We work closely together with clinical experts and geneticists in several labs in the Netherlands and across the world. Our research focusses on the complex genetics of human diseases such as Alzheimer’s Disease, Spinal Muscular Atrophy, Fragile X-syndrome, X-linked Dystonia Parkinsonism, Koolen-deVries syndrome, 1q21.1 deletion syndrome & Coffin-Siris syndrome.
In a more general view, research in the Evolutionary Neurogenomics group can be subdivided into two three main themes:
-
Investigating the role of new genes formed by segmental duplications in human brain development

The Neocortex has undergone a dramatic expansion during primate evolution. In our lab, we aim to characterize gene-regulatory networks that drive the earliest stages of cortical development with a special focus on aspects unique to humans. In work that was published in the scientific journal 'Cell' after many years of exciting discoveries, we revealed the existence of a cluster of human-specific NOTCH2NL genes, which are highly expressed in neuronal stem cells in the human brain. These human-specific genes were formed by segmental duplications, and their function in neuronal progenitor cells suggest these genes may have contributed to the evolutionary expansion of the human neocortex. We followed up this work by showing the appearance of functional variants in NOTCH2NL genes that exist in homo Sapiens, but not Neanderthals, that seem to be acting to refine the optimal dosage of NOTCH2NL protein that is presented to neural stem cells during early stages of neurodevelopment (Lodewijk et al., MBE 2020).
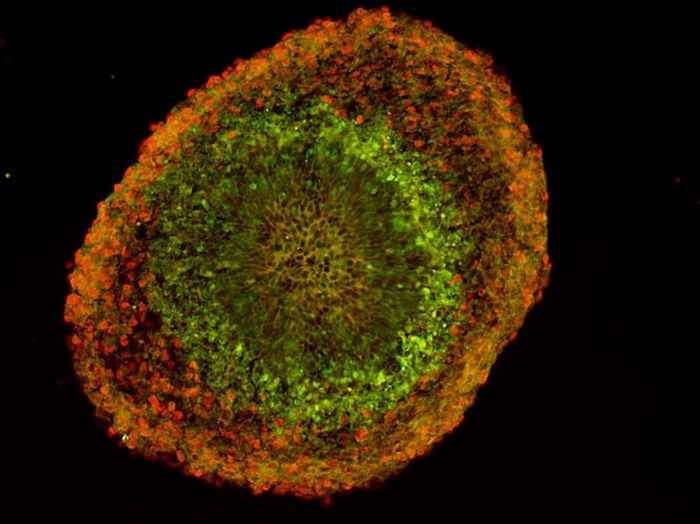
This gene-family is not alone. Many other multi-copy genes exist in our genome, some of which we know are highly expressed during early developmental stages of human cerebral organoid development. Since very recent gene-duplications result in two nearly identical and therefore nearly indistinguishable paralogous genes on the genome, most of these duplicated genes have escaped the attention.
It is becoming increasingly clear that big chromosomal rearrangements such as gene duplications may have had a significant impact on the evolution of species. Current research in my group focusses on neuronally expressed multi-copy genes and the impact of gene duplication events on the evolution of human neural gene-regulatory pathways.
-
The impact of retrotransposon invasions on the evolution of human gene regulatory networks
Retrotransposons are virus-derived mobile DNA elements that retained the capability to copy-paste themselves in the host genome, long after the initial attack of the virus and the insertion of the viral DNA into our genome. As a result, over 50% of the human genome is retrotransposon-derived, showing that mobile DNA elements have accumulated in our genome over the course of mammalian evolution.
Some recently emerged retrotransposon-families, such as LINE1 and SVA elements are still active in our genome and while new insertions can give rise to disease, they are an important source for genomic variability responsible for the continuing evolution of our genome. Even compared between the human and chimpanzee genomes, many thousands of species-specific retrotransposon insertions exist. The viral-like gene-regulatory properties of retrotransposons can have significant effects on gene expression when insertions of these ‘mobile promoters’ or ‘mobile enhancers’ happen near genes.
In previous work (Jacobs et al., 2014; Nature) we have found that two primate-specific KRAB zinc finger gene have recently evolved to repress the activity of SVA and L1PA retrotransposons. This study shows how our genome is in a continuous battle against retrotransposon invasions; an evolutionary arms race which explains the rapid expansion of KRAB zinc finger genes in primate genomes.
Intriguingly, the genome’s effort to silence retrotransposons also affects genes in the direct neighbourhood of their insertion sites, suggesting that both retrotransposons and KRAB zinc finger genes become integrated in pre-existing gene regulation pathways and may therefore be an important source for the evolution of gene-regulatory novelties.
Currently, our lab investigates the extent to which human-lineage specific retrotransposon insertions and the KRAB zinc fingers that evolved to repress them have contributed to the evolution of neural gene-regulatory pathways and how these new regulatory properties relate to human neurological disease.
-
The correlation between recent evolutionary changes in our genome and increased risk to human neurological diseases
What is emerging from our recent work and the work of others, is that loci that have evolved recently in primate and human evolution, have become genetically unstable (in case of segmental duplications) or added new regulatory features (in case of transposable elements). The impact of these genomic changes for how our genes are expressed, and how this can lead to disease under certain circumstances has become a central question in our lab, which has led us to investigate the genetic baiss of a number of neurodevelopmental and neurodegenerative diseases in more detail. For this we use our expertise in complex genomic loci and state of the art sequencing and genetic engineerging techniques to shed light on which challenges our species is facing as the flip side of the rapid expansion of the human neocortex in the last 2 million years.